Despite recent advances in therapeutic options for advanced melanoma patients, there is still an unmet medical need for melanoma patients with local recurrence, who are candidates for surgical resection of all metastases. These patients are at a high risk of further recurrences until the disease becomes inoperable (unresectable), or progresses to Stage IV (distant organ involvement), for which the prognosis is particularly grim.
NidlegyTM is a combination of the immunocytokines L19IL2 and L19TNF, which is being developed as intralesional treatment for patients with advanced locoregional melanoma and non-melanoma skin cancers. In preclinical melanoma tumor models, the combination of the two agents has shown striking anti-cancer responses achieving cures in all treated animals.
In a phase II clinical study conducted in stage III/IVM1a melanoma patients, NidlegyTM led to complete responses not only on the injected tumor lesions but also on the majority of non-injected
lesions (proving the onset of a systemic immune response induced after the intralesional injection of the product). A combination pack request for the use of NidlegyTM has been approved by EMA.
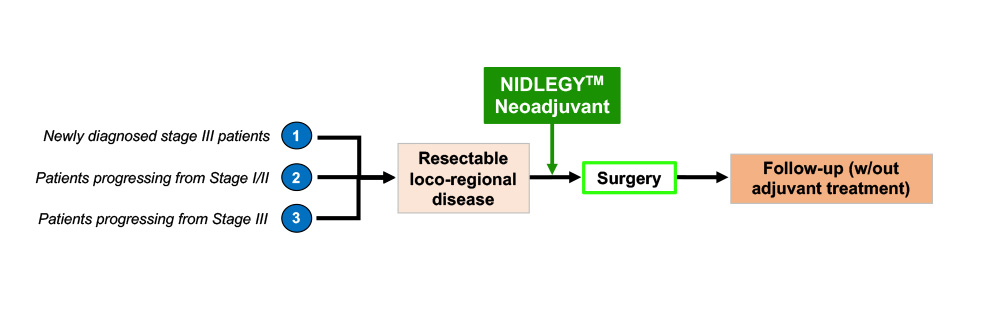
Based on these encouraging results, Philogen has started two phase III clinical trials (with the potential to support a marketing authorization application) in Europe and in the United States. Patients with resectable, locoregional melanoma will be treated with intralesional injections of NidlegyTM in the neoadjuvant setting (i.e., prior to surgery). In addition, Philogen has initiated a phase II clinical trial in the United States with NidlegyTM(intralesional) in combination with anti-PD-1 therapy (systemic) in patients that have progressed after anti-PD-1 therapy.
Inspired by the excellent performance of NidlegyTM in melanoma, Philogen has recently started two phase II clinical programs to investigate the efficacy and safety of the product in injectable, locally-advanced basal cell carcinoma and in other non-melanoma skin cancers.
ONGOING CLINICAL TRIALS
- Two randomized, controlled phase III registration trials for intralesional application of NidlegyTM as a neoadjuvant followed by surgery + eventual adjuvant treatments (standard of care) and compared to standard of care are currently ongoing in Europe (NCT02938299) and in the USA (NCT03567889) in patients with fully resectable stage IIIB/C melanoma.
- A phase II trial in non-melanoma skin cancer (i.e., high-risk patients with Basal Cell Carcinoma and Cutaneous Squamous-Cell Carcinoma) (NCT04362722).
ONGOING CLINICAL TRIALS
- Two randomized, controlled phase III registration trials for intralesional application of NidlegyTM as a neoadjuvant followed by surgery + eventual adjuvant treatments (standard of care) and compared to standard of care are currently ongoing in Europe (NCT02938299) and in the USA (NCT03567889) in patients with fully resectable stage IIIB/C melanoma.
Two phase II trials in non-melanoma skin cancers (i.e., high-risk patients with Basal Cell Carcinoma and other NMSCs) (NCT04362722 ; NCT05329792).
References
Weide et al. (2019) Cancer Immunol Immunother, 68, 1547-59
Kaplon and Reichert (2018) MAbs, 1-21
Weide, Neri and Elia (2017) Cancer Immunol Immunother, 66, 647-56
Danielli et al. (2015) Cancer Immunol Immunother, 64, 999-1009
Pretto et al. (2014) Cancer Immunol Immunother, 63, 901-10
Schwager et al. (2013) J Invest Dermatol, 133, 751-8
Lee et al. (2009) J Clin Oncol, 27, 3489-95
