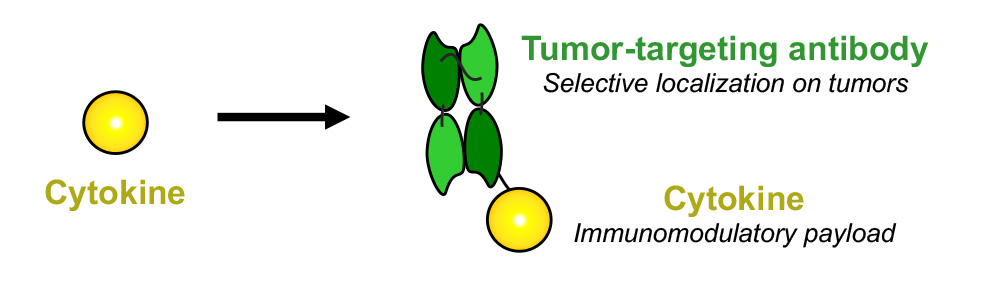
Antibody-Cytokine Fusions
We produce fully-human fusion proteins consisting of antibodies and cytokines (also called “immunocytokines”) in order to modulate the activity of the immune system at the site of disease. Cytokines are naturally produced proteins of the human body, which can either potently boost or suppress the activity of the immune system.
Some recombinant cytokines are products on the market, but their systemic administration at therapeutically active doses is often hindered by side effects. To overcome this limitation, we engineer innovative antibody-cytokine fusions to selectively socialize at the site of disease and to obtain an improved therapeutic index.
The cytokine payload is chosen depending on the nature of the disease. For example, pro-inflammatory cytokines such as TNF, IL2 or IL12 can be used in oncology, while anti-inflammatory cytokines such as IL10 can be used to treat inflammatory conditions.
From Cytokines to Immunocytokines
References
Corbellari et al. (2021) Mol Cancer Ther, 20, 859-71
Ongaro et al. (2020) Oncotarget, 11, 3698-3711
Weiss et al. (2020) Sci Transl Med, 7, 12(564):eabb2311
Neri (2019) Cancer Immunol Res, 7, 348-54
Hutmacher and Neri (2019) Adv Drug Deliv Rev, 15, 67-91
De Luca et al. (2017) Mol Cancer Ther, 16, 2442-51
Bootz and Neri (2016) Drug Discov Today, 21, 180-9
Kiefer and Neri (2016) Immunol Rev, 270, 178-92
Neri and Sondel (2016) Curr Opin Immunol, 40, 96-102
Hemmerle and Neri (2014) Int J Cancer, 134, 467-77
Hemmerle et al. (2014) Proc Natl Acad Sci USA, 111, 12008-12
Gutbrodt et al. (2013) Sci Transl Med, 5, 201ra118
Schwager et al. (2013) J Invest Dematol, 133, 751-8
Moschetta et al. (2012) Cancer Res, 72, 1814-24
Pasche and Neri (2012) Drug Discov Today, 17, 583-90
Pasche et al. (2012) Clin Cancer Res, 18, 4092-103;
Marlind et al. (2008) Clin Cancer Res, 14, 6515-24
Halin et al. (2003) Cancer Res, 63, 3202-10
Halin et al. (2002) Nat Biotechnol, 20, 264-9
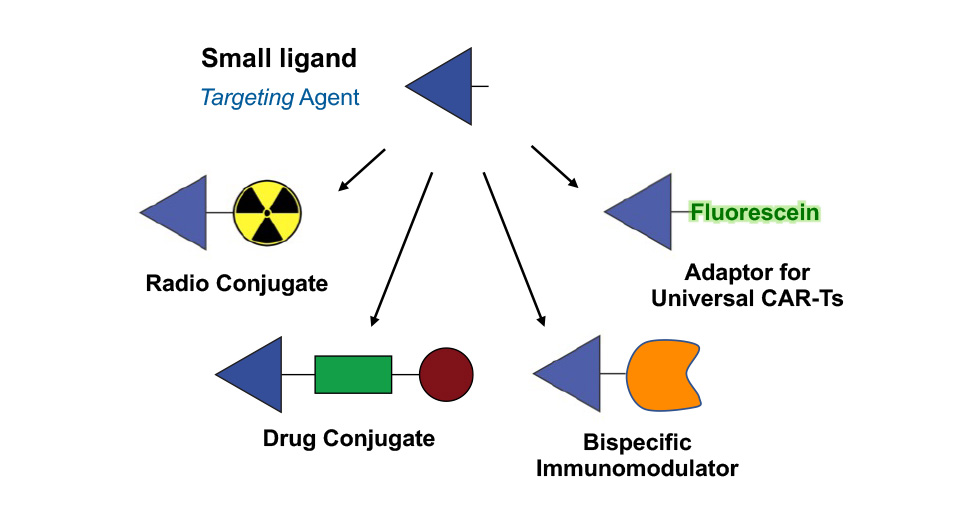
Small Molecule Therapeutics
Low molecular weight compounds can be used as alternatives to antibodies for tumor targeting applications. The advantages of small molecule therapeutics include a fast extravasation, a deep and homogeneous penetration into solid tumors, as well as faster development timelines.
Thanks to technological advances in the field of DNA-encoded chemical libraries, we are able to generate small molecule ligands with antibody-like properties featuring ultra-high affinity to their target antigen and long residence time at the tumor site.
Philochem has identified and patented several ligands against pathology-associated targets. These small organic ligands have been coupled to cytotoxic agents (small molecule drug conjugates), immunomodulatory payloads (bispecific immunomodulators), radionuclides (small molecule radio conjugates), or adaptors for universal Chimeric Antigen Receptor T cell therapy, resulting in several therapeutic and diagnostic programs.
References
Millul et al. (2021) PNAS, 118, 16, e2101852118
Millul et al. (2021) Mol Cancer Ther, 20, 512-22
Kulterer et al. (2021) J Nucl Med, 62, 360-5
Pellegrino et al. (2020) Bioconjug Chem, 31, 1775-83
Cazzamalli et al. (2018) Clin Cancer Res, 24, 3656-67
Cazzamalli et al. (2018) J Am Chem Soc, 140, 1617-21
Cazzamalli et al. (2017) J Control Release, 246, 39-45
Cazzamalli et al. (2016) Mol Cancer Ther, 15, 2926-35
Krall et al. (2016) J Nucl Med, 57, 943-9
Wichert et al. (2015) Nat Chem, 7, 241-9
Casi & Neri (2015) J Med Chem, 58, 8751-61
Minn et al. (2016) Oncotarget, 7, 56471-79
Yang et al. (2015) Oncotarget, 6, 33733-42
Krall et al. (2014) Chem Sci, 5, 3640-4
Krall et al. (2014) Angew Chem Int Ed, 53, 4231-5
Krall et al. (2013) Angew Chem Int Ed Engl, 52, 1384-1402
Müller et al. (2013) J Nucl Med, 54, 124-31
Ahlskog et al. (2009) Bioorg Med Chem Lett, 19, 4581-6
Dumelin et al. (2008) Angew Chem Int Ed Engl, 47, 3196-3201
