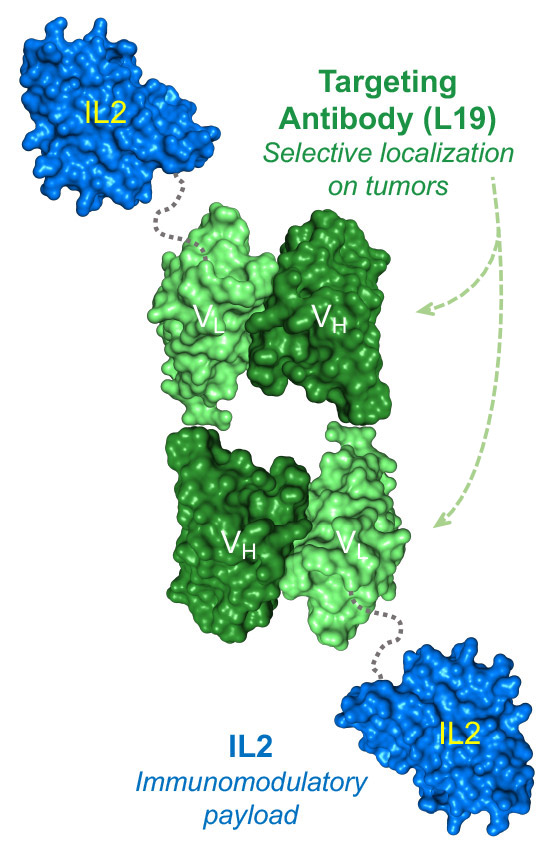
Darleukin (L19IL2) is a fully-human immunostimulatory product consisting of the human L19 antibody fused to the human cytokine interleukin-2 (IL2). (Recombinant IL2 is an approved biopharmaceutical.)
The fusion of IL2 to the L19 antibody, specific to the EDB domain of fibronectin results in a tumor-targeted product, which selectively localizes at the site of disease, while sparing healthy organs. Darleukin shows a strong anti-cancer activity, especially when combined with other therapeutic modalities. Mechanistically, the product kills cancer cells by activating tumor-specific T cells and NK cells at the site of disease.
Darleukin has been tested in numerous clinical studies, both as monotherapy and in combination with other drugs. Promising phase I results have been obtained especially when Darleukin was combined with stereotactic ablative radiotherapy (SABR).
In collaboration with the IMMUNOSABR European Consortium, Darleukin is currently being being studied in a phase II clinical trial, in patients with advanced non-small cell lung cancer, in combination with SABR and anti-PD-1 therapy .
ONGOING CLINICAL TRIALS
- An investigator-driven, controlled, multi-center phase II clinical trial with Darleukin immunotherapy in combination with Stereotactic Ablative Radiotherapy (SABR)in metastatic non-small cell lung cancer (IMMUNOSABR) (NCT03705403).
ONGOING CLINICAL TRIALS
- An investigator-driven, controlled, multi-center phase II clinical trial with Darleukin immunotherapy in combination with Stereotactic Ablative Radiotherapy (SABR)in metastatic non-small cell lung cancer (IMMUNOSABR) (NCT03705403).
References
Ongaro et al., (2020) Oncotarget, 11, 3698-3711
Weide et al. (2019) Cancer Immunol Immunother, 68, 1547-59
Zegers et al. (2015) Clin Cancer Res, 21, 1151-60
Rekers et al. (2015) Radiother Oncol, 116, 438-42
Rekers et al. (2015) Oncoimmunology, 4, p. e1021541
Danielli et al. (2015) Cancer Immunol Immunother, 64, 999-1009
Weide et al. (2014) Cancer Immunol Res, 2, 668-78
Schwager et al. (2013) J Invest Dermatol, 133, 751-8
Eigentler et al. (2011) Clin Cancer Res, 17, 7732-42
Eigentler et al. (2011) J Clin Oncol, 29, 2531
Johannsen et al. (2010) Eur J Cancer, 46, 2926-35
Schliemann et al. (2009) Blood, 113, 2275-2283
Carnemolla et al. (2002) Blood, 99, 1659-65
