Target Discovery
The development of targeted therapeutics crucially relies on the identification of target antigens, which are specifically expressed at the site of disease, and which are readily accessible for agents coming from the bloodstream.
In collaboration with its Swiss subsidiary Philochem and with ETH Zürich, Philogen scientists have pioneered chemical proteomics methods for the characterization of accessible
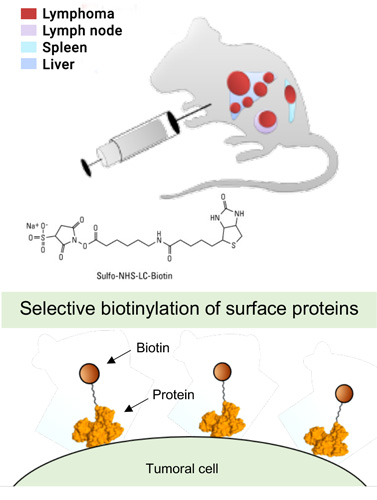
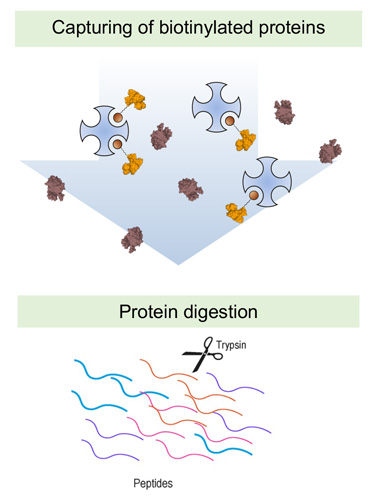
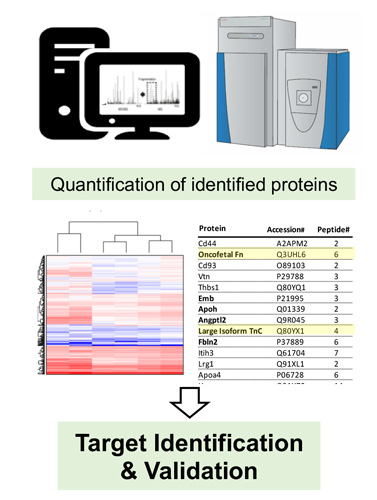
markers of pathology, which can be drugged by antibodies and small molecule ligands. In a simple setup, cell lines can be submitted to surface biotinylation followed by capture on streptavidin resin and mass spectrometric analysis of tryptic peptides.
In a more complex setup, in vivo biotinylation procedures of rodent models of pathology enable the chemical proteomic characterization of vascular proteins in health and in disease.
The technology has been extended to the ex vivo perfusion of surgically-resected human organs with cancer.
References
Elia et al. (2014) J Proteomics, 107, 50-5
Neri and Supuran (2011) Nat Rev Drug Discov, 10, 767-77
Fugmann et al. (2011) Kidney Int, 80, 272-81
Roesli et al. (2011) J Proteomics, 74, 539-46
Schliemann et al. (2010) Blood, 115, 736-44
Borgia et al. (2010) Cancer Res, 70, 309-18
Fugmann et al. (2010) Proteomics, 10, 2631-43
Rösli et al. (2008) Methods Mol Biol, 418, 89-100
Conrotto et al. (2008) Int J Cancer, 123, 2856-64
Castronovo et al. (2006) Mol Cell Proteomics, 5, 2083-91
Brack et al. (2006) Clin Cancer Res, 12, 3200-8
Scheurer et al. (2005) Proteomics, 5,2718-28
Rybak et al. (2005) Nat Methods, 2, 291-98
Neri and Bicknell (2005) Nat Rev Cancer, 5,436-46
Ligand Discovery
The discovery of specific binders to protein targets (antibodies or small organic ligands) is a crucial step in the discovery of new products at Philogen. We use encoded library technologies for the generation and screening of molecular repertoires of unprecedented size.
Our proprietary Antibody Phage Libraries comprise more than 100 billion members and enable the isolation of specific human monoclonal antibodies against virtually any antigen of interest.
References
Weber et al. (2014) PLoS One, 9, e100000
Cyranka-Czaja et al. (2012) Biochem Biophys Res Commun, 419, 250-5
Villa et al. (2011) MAbs, 3, 264-72
Sommavilla et al. (2010) J Immunol Methods, 353, 41-3
Villa et al. (2008) Int J Cancer, 122, 2405-13
Rybak et al. (2007) Cancer Res, 67, 10948-57
Brack et al. (2006) Clin Cancer Res, 12, 3200-8
Silacci et al. (2006) Protein Eng Des Sel, 19, 471-8
Silacci et al. (2005) Proteomics, 5, 2340-50
Giovannoni et al. (2001) Nucleic Acids Res, 29, E27
Viti et al. (2000) Methods Enzymol, 326, 480-505
Tarli et al. (1999) Blood, 94, 192-8
Pini et al. (1998) J Biol Chem, 273, 21769-76
Neri et al. (1997) Nat Biotechnol, 15, 1271-5
Antibody Phage Libraries
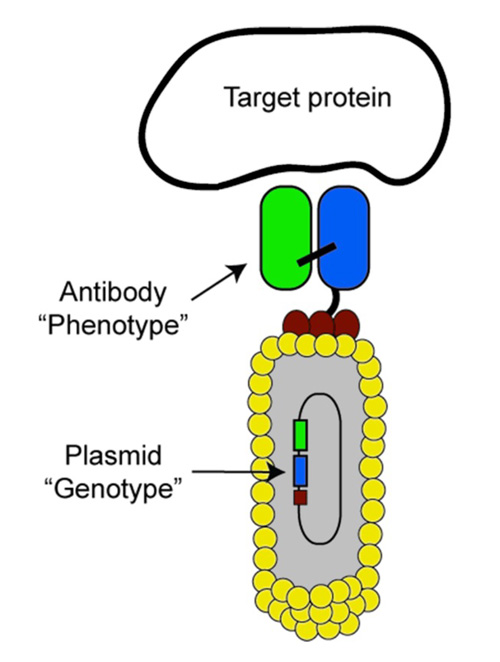
Picture adapted from Neri and Lerner (2018) Annu Rev Biochem., 87, 479-502
DNA-encoded Chemical Libraries
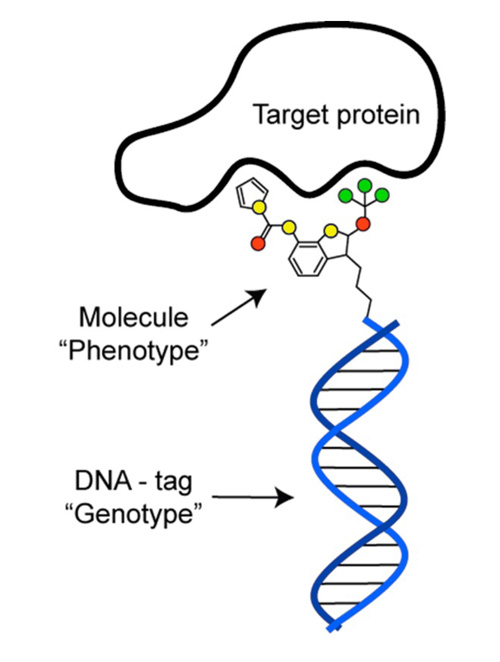
Picture adapted from Neri and Lerner (2018) Annu Rev Biochem., 87, 479-502
We have also been pioneers in the field of DNA-encoded Chemical Libraries, a revolutionary technology that allows the synthesis and screening of molecular repertoires with billions of chemical compounds.
Philogen´s encoded libraries of antibodies and small organic molecules are being used both for in-house discovery activities and for partnered programs. Our fully-owned Philochem subsidiary specifically focuses on encoded library technologies. For more information about possible partnerships, please see “Work with us”.
References
Favalli et al. (2021) Nat Chem, 13, 540-8
Sannino et al. (2020) ACS Comb Sci, 22, 204-12
Gironda et al. (2019) Org Lett, 21, 9555-58
Li et al. (2018) Nat Chem, 10, 441–48
Neri and Lerner (2018) Annu Rev Biochem, 87, 479-502
Favalli et al. (2018) ChemMedChem, 13, 1303–7
Zimmermann et al. (2017) Chemistry, 34, 8152–5
Bigatti et al. (2017) ChemMedChem, 12, 1748–52
Zimmermann and Neri (2016) Drug Discov Today, 21, 1828-34
Wichert et al. (2015) Nat Chem, 7, 241-9
Franzini et al. (2015) Angew Chem Int Ed, 54 3927-31
Samain et al. (2015) J Med Chem, 58, 5143-49
Leimbacher et al. (2012) Chem Eur, 18, 7729–37
Dumelin et al. (2008) Angew Chem Int Ed, 47, 3196-201
Scheuermann et al. (2008) Bioconj Chem, 19, 778-85
Mannocci et al. (2008) Proc Natl Acad Sci, 105, 17670-5
Melkko et al. (2007) Angew Chem Int Ed, 46, 4671-74
Melkko et al. (2004) Nat Biotechnol, 22, 568-74
GMP Manufacturing
In addition to our research facilities, we have operated a GMP manufacturing plant in Montarioso near Siena, Italy, since 2004. This site is subject to strict regulatory and manufacturing requirements.
We are currently completing the construction of a second GMP plant in Rosia, Italy, which will be used for the commercial production of our drugs.
Both Rosia and Montarioso are close to Siena, a city with a historically strong presence of successful biotechnology companies (also known as ‘‘Pharma Tuscany’’). The nature and location of our Italian facilities enable us to conduct GMP manufacturing and CRO drug development activities in an integrated manner.
Moreover, Philogen offers GMP manufacturing services for biotherapeutics to selected industrial and academic partners in the frame of collaborations. For more information, please see “Work with us”.
