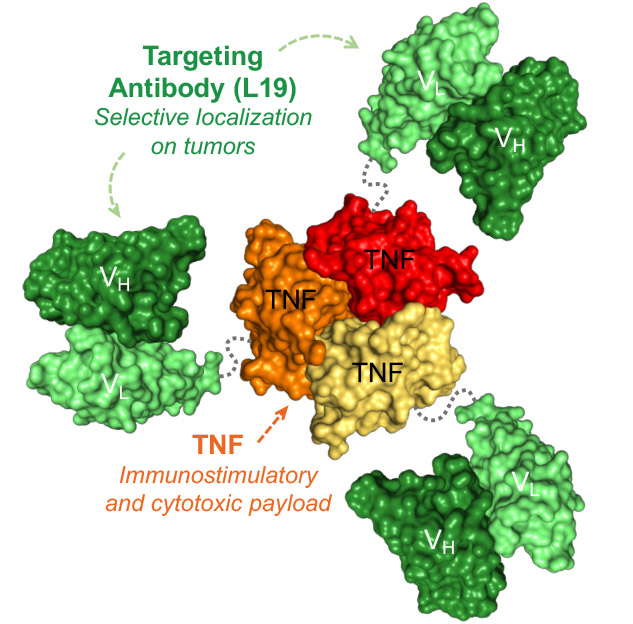
Fibromun (L19TNF) is a fully-human immunomodulatory product consisting of the L19 antibody and TNF (a strong pro-inflammatory cytokine). (Recombinant TNF has so far been approved only for certain clinical applications.)
Fibromun (L19TNF) is a fully-human immunomodulatory product consisting of the L19 antibody and TNF (a strong pro-inflammatory cytokine). (Recombinant TNF has so far been approved only for certain clinical applications.)
The fusion of TNF to the L19 antibody specific to the EDB domain of fibronectin results in a tumor-targeted product, which selectively localizes at the site of disease, while sparing healthy organs.
Fibromun has shown potent anti-tumor activity, both as single agent and in combination with other drugs, in several immunocompetent preclinical models inducing in most cases long-lasting complete responses.
Fibromun has previously been studied in clinical trials as monotherapy for systemic administration in patients with solid tumors and in melanoma patients using the Isolated Limb-Perfusion procedure.
In preclinical models of sarcoma, Fibromun has, when combined with doxorubicin or dacarbazine (which are standard therapies for first line and third line sarcoma, respectively), cured all treated animals . Based on the encouraging results observed in the phase Ib study conducted in patients with metastatic Soft Tissue Sarcoma, three clinical trials with registration potential have started in Europe and in the United States.
Fibromun has also shown promising activity in orthotopic mouse models of glioblastoma and in high-grade glioma patients (studies performed in collaboration with the University Hospital Zurich). On the basis of the promising results observed in a phase I/II monotherapy study in patients with recurrent glioma, two clinical studies with registration potential have recently been started in newly diagnosed and in recurrent glioblastoma patients, respectively.
ONGOING CLINICAL TRIALS
- Two pivotal clinical trials with Fibromun in combination with doxorubicin in metastatic Soft Tissue Sarcoma are ongoing in Europe (EudraCT:2016-003239-38) and in the USA (NCT03420014).
- A phase II clinical trial with Fibromun in combination with dacarbazine in pretreated Soft Tissue Sarcoma (EudraCT:2018-004104-19).
- A phase I/II clinical trial with Fibromun (monotherapy) in grade III/IV glioma at first relapse (NCT03779230).
- A phase I/II/IIb clinical trial with Fibromun in combination with radiotherapy and temozolomide in newly diagnosed glioblastoma (NCT04443010).
- A phase I/II clinical trial with Fibromun in combination with lomustine in grade III/IV glioma at first relapse (NCT04573192).
ONGOING CLINICAL TRIALS
- Two pivotal clinical trials with Fibromun in combination with doxorubicin in metastatic Soft Tissue Sarcoma are ongoing in Europe (EudraCT:2016-003239-38) and in the USA (NCT03420014).
- A phase II clinical trial with Fibromun in combination with dacarbazine in pretreated Soft Tissue Sarcoma (EudraCT:2018-004104-19).
- A phase I/II clinical trial with Fibromun (monotherapy) in grade III/IV glioma at first relapse (NCT03779230).
- A phase I/II/IIb clinical trial with Fibromun in combination with radiotherapy and temozolomide in newly diagnosed glioblastoma (NCT04443010).
- A phase I/II clinical trial with Fibromun in combination with lomustine in grade III/IV glioma at first relapse (NCT04573192).
References
Weiss et al., (2020) Sci Transl Med, 12 (564):eabb2311
Probst et al., (2019) Clin Cancer Res, 25, 698-709
Probst et al., (2017) Cancer Res, 77, 3644-54
Hemmerle and Neri (2013) Br J Cancer, 109, 1206-13
Papadia et al. (2013) J Surg Oncol, 107, 173-179
Spitaleri et al (2013) J Cancer Res Clin Onc, 139, 447-55
Balza et al. (2010) Int J Cancer, 127, 101-10
Schliemann (2009) Leuk Res, 33, 1718-22
Balza et al. (2006) Clin Cancer Res, 12, 2575-82
Borsi et al. (2003) Blood, 102, 4384-92
Halin et al. (2003) Cancer Res, 63, 3202-10
Santimaria (2003) Clin Cancer Res, 9, 571-9
Castellani et al. (2002) Am J Pathol, 161, 1695-1700
